Which Best Describes the Second Law of Thermodynamics
This module describes conduction convection and radiation heat transfer. Since second order reactions can be of the two types described above the rate of these reactions can be generalized as follows.

Solved Which Best Describes The Second Law Of Chegg Com
Students continuing to PHYS 4C will also need MATH 18 or 20F or 31AH.
. By the executive through decrees and. Processes occur spontaneously if and only if by their process the entropy change in the universe is greater than or equal to zero. R kA x B y.
Second Law of Thermodynamics. This is best answered by using the Carnot efficiency. The flux is the product of the number of turns in the coil and the flux associated with the coil.
Third law of thermodynamics. A few examples of second order reactions are given below. The formula of Faradays law is given below.
Faradays Second Law of Electromagnetic Induction. Faradays second law of electromagnetic induction states that. However no information about the direction of the process can be obtained by the application of the first law.
The ideal gas law is an equation of state that describes ideal gases. The module also explains how specific parameters can affect the rate of heat transfer. Volume 3 of 3 Module 3 - Fluid Flow This module describes the relationship between the different types of energy in a fluid stream through the use of Bernoullis equation.
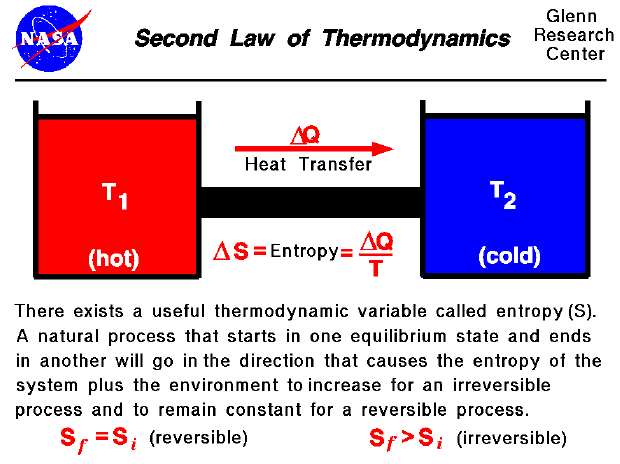
The second law of thermodynamics introduces the concept of entropy. The way that the second law describes the physics of this process is best understood in terms of the Austrian physicist Ludwig Boltzmanns interpretation of entropy as a measure of randomness. Zeroth law of thermodynamics.
This is the most common equation of state for gases. Examples of Second Order Reactions. The first law is used to relate and to evaluate the various energies involved in a process.
State-enforced laws can be made by a group legislature or by a single legislator resulting in statutes. Second Law of Thermodynamics By. The Second Law of Thermodynamics.
First law of thermodynamics. ΔS Total ΔS System ΔS surrounding. Entropy S is a quantity associated with the number of states in terms of either.
The second law of. U Internal energy V Volume and N i. Second law of thermodynamics.
Both heat transfer and work transfer are boundary phenomena. This equation of state relates a gass pressure volume temperature and mass and is very useful for describing how gases will behave in ideal conditions. Law is a system of rules created and enforced through social or governmental institutions to regulate behavior with its precise definition a matter of longstanding debate.
It is wrong to say total heat or heat content of a closed. S K Mondal 6. Continuation of PHYS 4A covering forced and damped oscillations fluid statics and dynamics waves in elastic media sound waves heat and the first law of thermodynamics kinetic theory of gases Brownian motion Maxwell-Boltzmann distribution second law of thermodynamics.
The induced emf in a coil is equal to the rate of change of flux linkage. Anyway the zeroth law of thermodynamics tells us about the state of thermal equilibrium of a system which describes the exchange of heat and the temperature of the system. I was today-years-old when I learned that zeroth was a word.
A 0064 kg of octane vapor MW 114 is mixed with091 kg of air MW 290 in the manifold of. The principles of thermodynamics are the principal laws governing thermodynamics. Where the sum of x and y which corresponds to the order of the chemical reaction in question equals two.
Kinda useful if you want to. The fact that heat energy is lost during cellular respiration is predicted by. The most important laws of thermodynamics are.
The First Law of Thermodynamics b. Chapter 6 Second Law of Thermodynamics Some Important Notes Regarding Heat Transfer and Work Transfer Heat transfer and work transfer are the energy interactions. The zeroth law of thermodynamics.
In differential form dQ Tds. The second law of thermodynamics also known as the Carnot principle after its discoverer in 1824 proves irrefutably that physical phenomena are irreversible especially when thermal changes occur. The second law of thermodynamics describes the nature of processes and chemical reactions as follows.
The Second Law of Thermodynamics. 1 For macroscopic systems above the thermodynamic limit these statements are both expressions of the second law of thermodynamics if the following expression is used for exergy. A few notable other ones are the Van der Waals and the Virial equation of state which both.
Second law of thermodynamics The entropy of an isolated system not in equilibrium will tend to increase over time approaching a maximum value at equilibrium. B U P R V T R S i μ i R N i displaystyle BUP_RV-T_RS-sum _imu _iRN_i 2 where the extensive quantities for the system are. It has been variously described as a science and the art of justice.
Which law that states Entropy of all perfect crystalline solids is zero at absolute zero temperature. The second law of thermodynamics is a general principle that goes beyond the limitations imposed by the first law of thermodynamics.
What Is The 2nd Law Of Thermodynamics Quora


No comments for "Which Best Describes the Second Law of Thermodynamics"
Post a Comment